Emission and Absorption Spectrum
Emission and Absorption Spectrum: Overview
This topic covers concepts such as Wavelength, Rydberg Constant, Hydrogen Spectrum, Lyman Series, Balmer Series, Paschen Series, Brackett Series, Pfund Series, Humphreys Series, etc.
Important Questions on Emission and Absorption Spectrum
The frequency of radio waves from a certain radio station is . Its wavelength is
The ratio of wavelengths of the last line of Balmer series and the last line of Lyman series is
Which of the following properties of atom could be explained correctly by Thomson model of atom?
Humphrey series in the hydrogen spectrum is obtained as a result of the jump of electrons from to .
The series of hydrogen that lies in the infrared region of electromagnetic spectrum is _____ series.
Briefly explain Humphrey series.
Name spectral series of hydrogen which lies in the infrared region of electromagnetic spectrum.
Which of the following is/are incorrect for Humphrey lines of hydrogen spectrum?
The shortest wavelength of the Lyman series of hydrogen spectrum is obtained during the transition of electrons between which of the following the shells:
Which of the following is a type of emission spectrum?
different wavelength may be observed in the spectrum from a hydrogen sample if the atoms are excited to states with principal quantum number ? The value of is
An electron in a hydrogen atom falls from an orbit with principal quantum number to What is the wavelength of the emitted radiation ? (where denotes the Rydberg constant).
If the limit wavelength of the Balmer series is , then the series limit wavelength of the Pfund series is what?
If the series limit frequency of the Lyman series is , then what is the series limit frequency of the Pfund series?
Who did experimental discovery of the Pfund series?
Pfund series will come after Lyman series.
What is order of pfund series in atomic sptectra?
An electron of a stationary hydrogen atom passes from the fifth energy level to the ground level. The velocity that the atom acquired as a result of photon emission will be (m is the mass of the electron, R, Rydberg constant and h Planck's constant)
What is the number of spectral lines emitted when the electron of a hydrogen atom moves from to state?
Pertain to the following statement and figure.
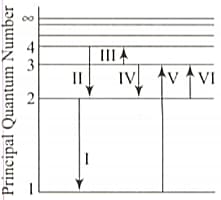
The figure above shows an energy level diagram of the hydrogen atom. Several transitions are marked as ... The diagram is only indicative and not to scale.
In which transition is a Balmer series photon absorbed?
